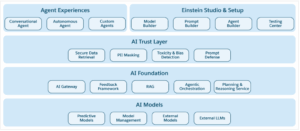
A newly developed catalyst built by precisely replacing cerium atoms at specific locations on a cerium oxide surface is nine times better than control catalysts used to oxidize carbon monoxide. Built by researchers at the Lawrence Berkeley National Laboratory (Berkeley Lab), the research effort includes other research institutes like the Oak Ridge National Laboratory in the US and the National Synchrotron Radiation Research Center in Taiwan.
Catalysts are substances that aid a chemical reaction by increasing its pace or reducing the activation energy without being consumed. At the end of the reaction, the catalyst remains available to facilitate more reactions and is therefore a favorite of the chemical industry as it enables cost-effective and efficient reactions.
Not just speed and efficiency, selectivity is also a major attribute of a catalyst as it facilitates specific reactions, improving their speed and reducing wastage. Conventionally, catalysts have been fabricated by processing hundreds of atoms at a time. However, researchers have been working on more selective approaches where individual atoms are carefully manipulated to increase the selectivity of catalyst behavior.
Replacing cerium with platinum
Researchers at Berkeley Lab developed a new process that allows the selective replacement of a cerium atom with a platinum one. The selectivity offered by this process is quite high, and the researchers equated it to mounting a diamond on top of the ring structure.
In the following step, the platinum-cerium structure is supplied with hydrogen molecules, where new bonds with cerium are made. To verify if these actions had specific impacts, the researchers also made a control catalyst that featured a platinum atom but was located randomly and not supplied with hydrogen molecules.

Impact
To verify the performance of the two catalysts, the researchers carried out two separate reactions. One involved the oxidation of carbon monoxide (CO) to form carbon dioxide (CO2). The other involved the removal of hydrogen from propane to make propylene, which serves as a raw material for the plastic industry.
In the first reaction, the catalyst oxidized the CO molecules up to nine times faster than when compared to the control catalyst. In the second reaction, the catalyst was 2.3 times more selective when converting propane to propylene.
“Our study provided deep insights into the chemical structure, reaction mechanisms, and performance of these advanced catalysts,” said Ji Su, a research scientist at Berkeley Lab, in a press release.
“It sets the stage for a new era in superior catalyst design, with the potential to dramatically improve the production efficiency of a wide range of chemical industry and environmental applications.”
While the research provides a comprehensive picture of how new catalysts can be fabricated, are structured, and have their chemical and physical properties, these developments would not have been possible without collaboration with multiple institutes in the US and outside.
The Advanced Light Source and Molecular Foundry, two premier user facilities developed by the Office of Science at the Department of Energy in the US, helped create high-resolution images of the platinum inserted on the catalyst’s surface and conducted simulations for the atomic structures and reaction pathways.
While the Oak Ridge National Laboratory helped characterize the bonding of cerium and hydrogen, the National Synchrotron Radiation Research Center in Taiwan helped the researchers understand the crystal structure better.
The research findings were published in the journal Science.